Biocompatible ceramic bone graft substitute “APAERAM” was created by our own unique technology.
Approved products, models and indications may differ from country to country.
Please contact us for detail.
Feature of APACERAM
Biocompatibility
APACERAM consists of synthetic hydroxyapatite 〔Ca10(PO4)6(OH)2〕that is an inorganic component of human bone. It has excellent biocompatibility and biological safety.
Osteoconductivity
APACERAM has macro pores and micro pores in the structure. Macro pores are effective for new bone formation, while micro pores provide interconnectivity of the pores.
Wide variation
Wide varieties of the models are available with various mechanical strength and porosity depending on the application sites and intended use.
Type-B (block form) and Type-G (granule form)
Feature of APACERAM porous materials
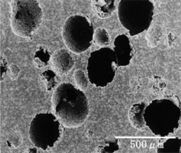
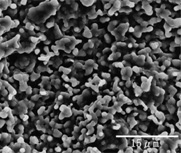
SEM images of APACERAM.
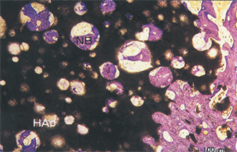
Undecalcified sample of APACERAM that has been placed in the femur of adult canine (3 months after implantation).
New bone was observed intruding into pores of APACERAM.
Feature of APACERAM (dense material)
SEM image of the dense material (surface
High density sintered body consisting of closely packed particles of 0.3~0.5μm.
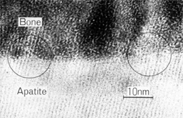
TEM image of the interface between APACERAM (dense material) and the bone
【Photo】 Dr. Makoto Ogiso (Tokyo Medical Dental University)
Crystalline particles are continuously emerging in the same direction at the interface between bone and APACERAM. It confirms complete chemical bonding between bone and APACERAM. (TEM image:2 months after implantation)
Porosity and mechanical strength of APACERAM
| Porosity (%) |
3 point bending strength (MPa) |
Compressive strength (MPa) |
| 0 |
220 |
730 |
| 5 |
90 |
540 |
| 15 |
50 |
240 |
| 30 |
27 |
175 |
| 40 |
16 |
65 |
| 50 |
10 |
30 |
| 55 |
9 |
20 |
| 60 |
7 |
15 |
Product Line-up (Type-G and Type-B)
A variety of porosity and shapes are available depending on the intended use and implantation site, including versatile blocks, granules and various spacers.
Type-AX
Type-AX is a hydroxyapatite bone graft substitute of ultra-high porosity of “Triple pore structure”.
“Triple pore structure”
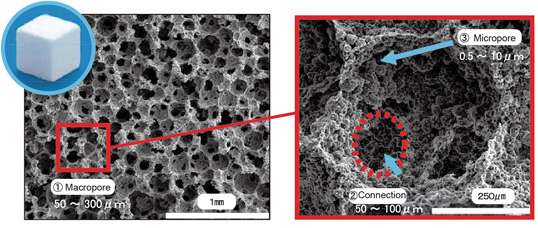
Triple pore structure:① Spherical macro pores, ② Considerable interconnecting pores, ③ micro pores among the spherical particles
Integration to bone tissue※
APACERAM Type-AX implanted in the defect Ø4mm × 6mmL in the iliac of the beagle (radiography).
APACERAM Type-AX implanted in the defect Ø4mm × 6mmL in the iliac of the beagle (radiography).
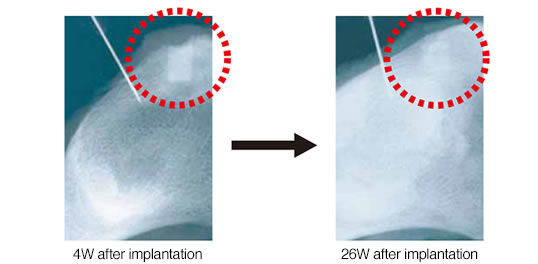
In 26th weeks after implantation, boundaries around the material became vague showing progress of new bone formation.
※To become almost same situation as bone
tissue in terms of structures and functions.
Early bone formation
Interconnecting pore structure and porous inner wall structure of the pores enable early bone formation.
a) APACERAM Type-AX implanted in the defect Ø4mm × 6mmL in the iliac of the beagle (Toluidine blue staining).
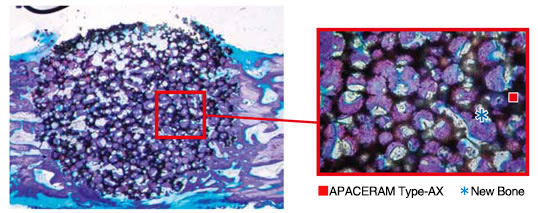
In 4 weeks after implantation, new bone formation was observed all over the implanted site with active new bone in the pores.
b)Implantation of APACERAM Type-AX in the defect Ø4mm × 12mmL in the iliac of the beagle. (Hematoxylin Eosin staining)
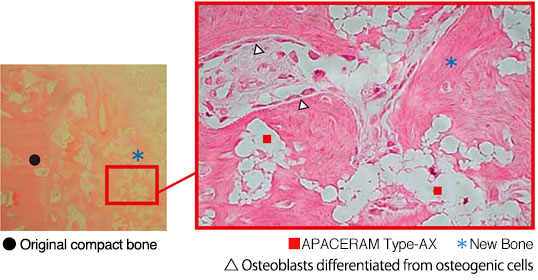
In 4 weeks after implantation, new bone formation was observed in the pores and osteoblasts were found on the surface of the newly formed bone.
Product Line-up (Type-AX)