Hydroxyapatite (Ca10 (PO4) 6 (OH)2) is a calcium phosphate compound and is the major inorganic component of human bones and teeth. It has high biocompatibility and is used as a biomaterial such as artificial bone.
History of Hydroxyapatite Chromatography
Hydroxyapatite chromatography was first developed at Uppsala University in Sweden in 1956.
Until the 1980s, hydroxyapatite as a column media was used by researchers to synthesize crystals from phosphoric acid and calcium, and the conditions for its synthesis were not fixed. Because the size and shape of the synthesized particles were inhomogeneous and irregular, when the particles were packed in the column and flowed at a high flow rate, the particles were broken and the column was clogged, making repeated use impossible.
History of Hydroxyapatite Chromatography by HOYA Technosurgical
In the late 1980s, HOYA Technosurgical (Asahi Optical Co., Ltd. at that time) developed a technology to make Hydroxyapatite crystals into spherical ceramics particles and applied it to chromatography media.
Spherical ceramics particles overcome the drawbacks of conventional Hydroxyapatite flake-like particles, such as low durability, low flow rate, non-repeatability, and differences between products lots.
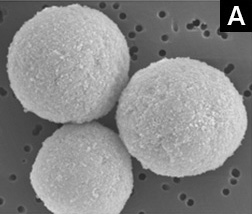
A: Spherical particles manufactured by HOYA Technosurgical
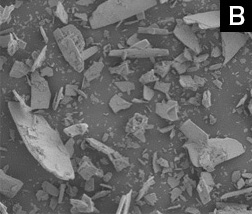
B: Conventional flake particles
Characteristics of Hydroxyapatite Products of HOYA Technosurgical
- product stability
- small differences between lots
- High purity manufactured in GMP grade factories
- supply stability
Hydroxyapatite media manufactured by HOYA Technosurgical are used in the manufacture of antibody drugs and vaccines by pharmaceutical companies around the world due to their high impurity removal and purification performance.
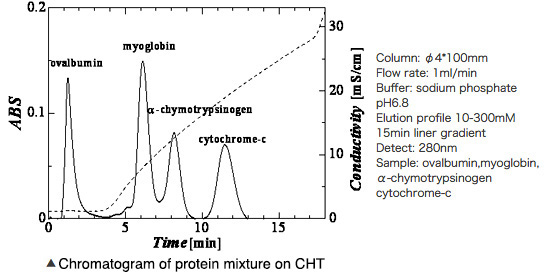
Hydroxyapatite Chromatography Products and Protein Separation Pattern on Hydroxyapatite column
Above: Hydroxyapatite chromatography media products: CHT
Below: elution profile of four kinds of mixture proteins with liner gradient of phosphate buffer on CHT column
Left: Hydroxyapatite chromatography media products: CHT
Right figure: elution profile of four kinds of mixture proteins with liner gradient of phosphate buffer on CHT column
*CHT is a chromatography media product manufactured by HOYA Technosurgical and sold by Bio-Rad Laboratories, Inc.
Worldwide distributor
Bio-Rad Laboratories, Inc. Life Science Group
TEL: 1-510-741-1000
1-800-4-BIORAD (1-800-424-6723)(U.S. only)
www.bio-rad.com
Patent of Hydroxyapatite Chromatography by HOYA Technosurgical
| patent number |
Title of the invention |
| US7,740,820 |
METHOD OF PRODUCING FLUOROAPATITE, FLUOROAPATITE, AND ADSORPTION APPARATUS |
| US8,481,770 |
SEPARATION METHOD
|
| US8,609,055 |
METHOD OF PRODUCING FLUOROAPATITE, FLUOROAPATITE, AND ADSORPTION APPARATUS |
| US9,266,086 |
COATED PARTICLES, METHOD OF PRODUCING COATED PARTICLES AND ADSORPTION APPARATUS |
| US10,407,306 |
METHOD OF PRODUCING FLUOROAPATITE POWDER, FLUOROAPATITE POWDER, AND ADSORPTION APPARATUS |
| US10,710,050 |
POWDER, METHOD OF PRODUCING POWDER, AND ADSORPTION APPARATUS |
| US11,161,090 |
POWDER, METHOD OF PRODUCING POWDER, AND ADSORPTION APPARATUS |
| application number |
Title of the invention |
| US16/549,199 |
POWDER, METHOD OF PRODUCING POWDER, AND ADSORPTION APPARATUS |
| US17/474,356 |
POWDER, METHOD OF PRODUCING POWDER, AND ADSORPTION APPARATUS |
References
Virus
- Kurosawa Y, Kurane I and Yamamoto A. Observation of Japanese encephalitis virus particles on ceramic hydroxyapatite by scanning electron microscopy. Med Biol. 153 (12), 607-610, 2009.
- Kurosawa Y, Saito M, Kobayashi S and Okuyama T. Purification of dengue virus particles by one-step ceramic hydroxyapatite chromatography. World Journal of Vaccines. 2, 155-160, 2012. DOI: 10.4236/wjv.2012.23020
- Kurosawa Y, Yamamoto A, Kurane I and Nakayama M. Development of a purification method for Japanese encephalitis virus particles using ceramic hydroxyapatite chromatography. Med Biol. 156, 410-416, 2012.
- M. Saito, Y. Kurosawa and T. Okuyama. Scanning electron microscopy-based approach to understand the mechanism underlying the adhesion of dengue viruses on ceramic hydroxyapatite columns. PLoS One 8(1), e53893, 2013. DOI: 10.1371/journal.pone.0053893
- Kurosawa Y, Saito M, Yoshikawa D and Snyder M. Mammalian virus purification using ceramic hydroxyapatite. Tech Note Bulletin 6549, Bio-Lad Laboratories, Inc. 2014.
http://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6549.pdf
- Minkner R, Baba R, Kurosawa Y, Suzuki S, Kato T, Kobayashi S and Park EY. Purification of human papillomavirus-like particles expressed in silkworm using a Bombyx mori nucleopolyhedrovirus bacmid expression system. J Chromatogr B. 1096, 39-47, 2018.
https://doi.org/10.1016/j.jchromb.2018.08.007
- Kurosawa Y, Khandelwal P, Yoshikawa D, and Snyder M. Single-step influenza and dengue virus purification with mixed-mode CHT ceramic hydroxyapatite XT media. Bulletin 7115, Bio-Rad Laboratories, Inc. 2018.
https://www.bio-rad.com/webroot/web/pdf/psd/literature/Bulletin_7115.pdf
Antibody
- Moro A, Yoshitake T, Ogawa T and Ichimura T. Single-step purification of pepsin-derived monoclonal antibody fragments from crude murine ascitic fluids by ceramic hydroxyapatite high-performance liquid chromatography. J Biochem. 144(6), 733-739, 2008. DOI: 10.1093/jb/mvn128.
- Saito M, Kurosawa Y and Okuyama T. Purification of anti-Japanese encephalitis virus monoclonal antibody by ceramic hydroxyapatite chromatography without Proteins A and G. Hybridoma (Larchmt). 31(1), 68-71, 2012. DOI: 10.1089/hyb.2011.0072.